

It is one of the alkali metals in the periodic table that possesses a single valence electron in the outer electron shell. It is silvery when first cut, but as soon as it comes into contact with oxygen, it starts to tarnish and turn gray. When cut, potassium’s silvery color changes to gray | source: Science Stock Footage The generated sodium chloride is continually removed from beneath the reaction zone. The lighter boiling potassium is separated to the required degree of purity using a fractionating column located above the reaction zone. Īn equilibrium vapor of sodium and potassium is produced when molten potassium chloride is added to a tightly packed column and brought into contact with rising sodium vapors in a reaction zone. Metallic potassium and sodium-potassium alloys (NaK) are produced by reacting high temperature sodium at atmospheric pressure with molten potassium chloride. About 70% of the potash produced worldwide in 2021 was produced by three nations Belarus, Russia, and Canada. Canada is the world’s largest potash producer, accounting for 31% of world production in 2021. In 2021, it was predicted that the world would produce about 71.9 million tonnes of potash. Most commercial potassium compounds are produced by electrolysis from soluble potassium compounds, such as carnallite, sylvite, polyhalite, and langbeinite, which are discovered in old lakes and seabeds. Potassium is abundant in leafy greens, beans, nuts, dairy products, milk, chocolate, bananas, and avocados. All fruits, vegetables, meat, and fish contain potassium. Numerous additional saline bodies of water are rich in potassium, with the Dead Sea having an estimated potassium chloride level of 1.7%. It is the seventh most abundant element in the Earth’s crust, making up around 2.6% of its weight. Because of this, the majority of potassium is found in the Earth’s crust as clays and feldspars. Potassium is not found in its elemental form in nature because it reacts so easily with water. OccurrenceĪbout 2.6 percent of the mass of Earth’s crust is potassium | source: The Layered Earth Despite a list of names in many languages, Gilbert and Klaproth’s names Natrium and Kalium were eventually chosen by Germanic countries, while Davy and Gay-Lussac/Thénard’s names Sodium and Potassium were accepted by English and French-speaking countries. Berzelius shortened Potassium and Sodium as Po and So in 1813. Gehlen afterwards suggested Kalin(um) and Natrin or Natrinmetall in 1808. He undoubtedly used Klaproth’s suggestion from 1797. Davy’s research findings were published in German by Ludwig Wilhelm Gilbert. The metals were initially referred to as métal de potasse and métal de soude, and later as Potassium and Sodium by Gay-Lussac and Thénard, who also studied the alkalis. Humphry Davy gave the element the name potassium, which he derived from the word potash after he separated the pure element using electrolysis in 1807. Martin Heinrich Klaproth was the first to differentiate between the two alkalis, suggesting the names kali for vegetable alkali and natron for mineral alkali. Sir Humphry Davy | source: Chemistry World Both substances were known as natron in Europe. Potash and soda (sodium carbonate, Na 2CO 3) were both referred to as alkali by the Arabs, and there was no distinction between them from antiquity until the Middle Ages. The chemical symbol K derives from kalium, the Mediaeval Latin for potash, which may have evolved from the Arabic term qali, meaning alkali.
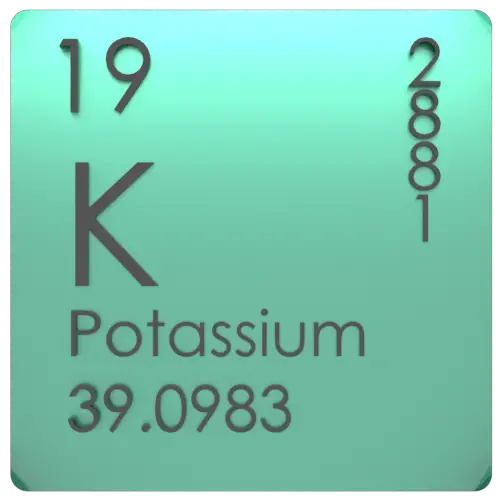
The English term first appeared in 1648 and is a borrowed translation of the Dutch word potaschen. Potassium was first derived from potash, meaning plant ashes, from which it received its name.
#Potassium element card how to
Learn how to find: Potassium valence electrons Learn how to find: Potassium protons neutrons electrons Potassium location on periodic tablePotassium is found in the first column of the periodic table below the sodium element.


 0 kommentar(er)
0 kommentar(er)
